The test is the day after tomorrow. It definitely feels farther away than it is. Seeing as I spent most of my weekend studying and being a nerd I think I will do ok.*knocks on every piece of wood in a ten foot radius*. Anyway, to prepare, I have done the practice quizzes may a time, and have done the big packets.... so all of the packets. It is definitely making sense, so at this point it is simply firming up the details and doing a lot of practice. (useless non chemistry info coming up so stop if you want)That is pretty much it. Im ready for summer guys. I got the lifeguard job I was hoping for a t the summer camp I love and I just want the time to fly so I can be there... Thats it. Thanks for listening if you made it. Have a wonderful week friends.
Monday, March 14, 2016
Tuesday, March 8, 2016
Helpful Links
Crash Course Help
Crash Again And Again
Another One(In this one, he touches on things we haven't learned yet in the first lesson, and it will hurt your brain... don't do the whole thing... IT'S A TRAP)
Good Luck!!!
Crash Again And Again
Another One(In this one, he touches on things we haven't learned yet in the first lesson, and it will hurt your brain... don't do the whole thing... IT'S A TRAP)
Good Luck!!!
First Lesson
Our first lesson was of course overwhelming, so I will try to help unconfuse myself and you.
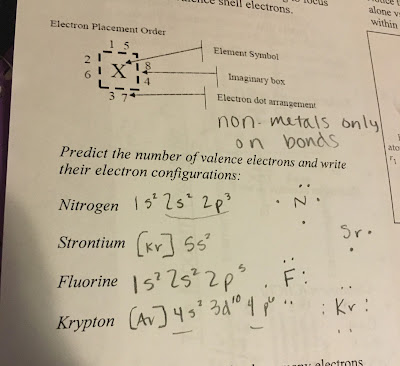
This lesson was about covalent bonds and how we structure them in models. The Lewis model that we learned only diagrams the valence electrons found in the s and p blocks. The most common covalent bond is between two non-metals.
This lesson was about covalent bonds and how we structure them in models. The Lewis model that we learned only diagrams the valence electrons found in the s and p blocks. The most common covalent bond is between two non-metals.
The dots on the outside of the diagram(designated on the electron placement order diagram as numbers 1-8) represent the valence electrons for each atom, and therefore represents the amount of electrons needed to be stable and how many bonds the atom can make. Each atom wants to have 8 electrons to be happy, so this is called the octet rule. No more than eight in the outer shell. AAAAnnndd since it's chemistry there are exceptions. Hydrogen and Helium can only hold two electron and therefore make one bond(each bond involves two electrons so split the number of electrons in half to get the number of bonds). Boron and Beryllium are pretty messed up but were sticking with 3 bonds for boron and idek for beryllium. Also, remember that elements in the 3rd primary energy level and beyond have the d sublevel, which means these atoms can expand(yet it is not shown on the Lewis diagram... just know that they do that).
The distance between two nuclei is called bond length(that one is pretty easy). In reality the orbitals of each atom get all up I each others business so instead of being able to just add the radii of each atom together to get the bond length theres cool math that we aren't learning(at least not in the first lesson) so if a question is asked about it just add the two together and pick the answer choice that is less than that.(phew I hope you guys are getting this).
The next thing on the notes is called Bond energy graphs that are to be read right to left.
On the far right is when the two atoms are not all up in each others business. Picture these atoms as magnets with the same poles trying to touch. They don't want to touch each other, but when you force them together they touch, which is the Same with these atoms. Something needs to put energy in for these guys to get together. As more energy is added, more potential energy builds up waiting to be released. so the more negative the y axis gets, the more potential energy there is going on in the bond. the far left is the potential energy being released and trickled off.
Guys, without a doubt this is helping me understand better than I expected, but this post is getting very long, so I am going to stop here and go to bed sometime soon. I bolded most of the important info so if you didn't want to read all of it i totally get it. If you did read it, I am clapping for you... because it was a lot. Alright, I hope your brains don't explode. Have a wonderful rest of your third quarter and good luck with your grades
Sunday, March 6, 2016
I Liked This Unit
Well since I didn't have much else to post about I decided to share with you how I liked this unit. First it wasn't as difficult as the past two units so my brain and my grade are very happy about that. Second, I felt that electron configurations were similar to a puzzle we had to figure out and that was enjoyable. Lastly, the whole unit was a lot of memorization which I am fair at so overall I liked. That is all.
Test Reflection
The test was on friday, and I felt good on it. I am waiting for the grade to be put in, because like always I need a good grade. Studying went well and I felt like I knew what i was doing. Ugh, the suspense is killer.
I hope everyone did well, and I hope you have a stress free weekend.
Subscribe to:
Comments (Atom)