Well, with 8 days left, I am really ready for summer to be here. Also, in complete chemistry fashion, we have studying and work up until the very end. This class has been challenging, rewarding, and really fun. I'm excited to get the last test done, and I think it will feel surreal. Good luck to everyone as we learn fancy gas laws and air bags. It's been fun, and I wonder what will happen to the group chat.
Monday, May 9, 2016
Links!!
The Ideal Gas Law
We only have 8 days left, friends. I know it feels like torture, but we can do it!
Airbag Lab
It was actually really cool. I remember from last year, the pre-ap kids talking about this lab, and i'm pretty sure they were freaking out way more than we were. once we understood what was going on, it was relaxed and fun. Mine went really well. I got a bigger bag, and it was perfectly filled. I really liked.
Thursday, May 5, 2016
Gas Laws Quiz
Well, it wasn't the worst quiz I have ever taken. There were as many concept questions as Frank said there would be. I actually thought it went well. I guess we'll see. Hoping to get the grade back quickly.
Hope everyone did well.
Hope everyone did well.
Thursday, April 28, 2016
Energy Lab
Wednesday, April 20, 2016
Energy and Phase Change Study Places
Crash Course Fun!
We know by now that crash course is helpful
But wait theres more
Here's some Khan Academy
link 5
Link 6
If you need a review of our unit, here are the resources I found to help you out with that. Good luck!
We know by now that crash course is helpful
But wait theres more
Here's some Khan Academy
link 5
Link 6
If you need a review of our unit, here are the resources I found to help you out with that. Good luck!
Friday, April 15, 2016
Boat Races
Well, for the look of the boat that my partner brought in, I honestly thought my boat would do better. I wasn't going for the win, and the boat made it, so I guess that is al that matters. The first time around our little sail boat kept getting stuck on the side so it wasn't moving fast. At one time, it stopped completely and went backwards. It was funny, but I was scared that it wouldn't finish. the second time I put little toothpicks in the side of it to keep it from hitting the side, and it went at a solid time of 45 seconds. Hey, I'll take it.
Here the boat that I named the struggle boat. Hope everyone else's went well.
Sunday, April 10, 2016
The Making of Biodiesel

Heyo friends, The next step in our biodiesel unit was to make our biodiesel for our boat races later next week. It was pretty cool, we got to boil some stuff and then do a cool viscosity test on some other liquids. I hope that my partner and I's biodiesel turns out okay, because I would really like to end this year on a high note for chemistry.
Some links about biodiesel and the effects of petroleum on the Earth:
Climate Change!
Info
A Website with some cool stuff
More Stuff
Yay! Stuff
This one shows a bit of the other side! Have Fun!
Tuesday, April 5, 2016
Biodiesel Viedo
I am going to tell you a secret. I have gym class before chem(ha ha, funny but not the secret), and because I change quickly that say, my shirt was on inside out when we filmed. Also, you totally can;t tell so I was very lucky. So my complete dorkiness aside, the video went well. As can be expected, it is embarrassing, but it will get the job done. Now on to the lab and put put boat section of this unit. Wish me luck.
Monday, March 14, 2016
Test Prep
The test is the day after tomorrow. It definitely feels farther away than it is. Seeing as I spent most of my weekend studying and being a nerd I think I will do ok.*knocks on every piece of wood in a ten foot radius*. Anyway, to prepare, I have done the practice quizzes may a time, and have done the big packets.... so all of the packets. It is definitely making sense, so at this point it is simply firming up the details and doing a lot of practice. (useless non chemistry info coming up so stop if you want)That is pretty much it. Im ready for summer guys. I got the lifeguard job I was hoping for a t the summer camp I love and I just want the time to fly so I can be there... Thats it. Thanks for listening if you made it. Have a wonderful week friends.
Tuesday, March 8, 2016
Helpful Links
Crash Course Help
Crash Again And Again
Another One(In this one, he touches on things we haven't learned yet in the first lesson, and it will hurt your brain... don't do the whole thing... IT'S A TRAP)
Good Luck!!!
Crash Again And Again
Another One(In this one, he touches on things we haven't learned yet in the first lesson, and it will hurt your brain... don't do the whole thing... IT'S A TRAP)
Good Luck!!!
First Lesson
Our first lesson was of course overwhelming, so I will try to help unconfuse myself and you.
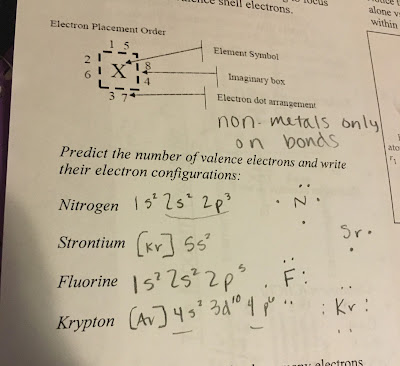
This lesson was about covalent bonds and how we structure them in models. The Lewis model that we learned only diagrams the valence electrons found in the s and p blocks. The most common covalent bond is between two non-metals.
This lesson was about covalent bonds and how we structure them in models. The Lewis model that we learned only diagrams the valence electrons found in the s and p blocks. The most common covalent bond is between two non-metals.
The dots on the outside of the diagram(designated on the electron placement order diagram as numbers 1-8) represent the valence electrons for each atom, and therefore represents the amount of electrons needed to be stable and how many bonds the atom can make. Each atom wants to have 8 electrons to be happy, so this is called the octet rule. No more than eight in the outer shell. AAAAnnndd since it's chemistry there are exceptions. Hydrogen and Helium can only hold two electron and therefore make one bond(each bond involves two electrons so split the number of electrons in half to get the number of bonds). Boron and Beryllium are pretty messed up but were sticking with 3 bonds for boron and idek for beryllium. Also, remember that elements in the 3rd primary energy level and beyond have the d sublevel, which means these atoms can expand(yet it is not shown on the Lewis diagram... just know that they do that).
The distance between two nuclei is called bond length(that one is pretty easy). In reality the orbitals of each atom get all up I each others business so instead of being able to just add the radii of each atom together to get the bond length theres cool math that we aren't learning(at least not in the first lesson) so if a question is asked about it just add the two together and pick the answer choice that is less than that.(phew I hope you guys are getting this).
The next thing on the notes is called Bond energy graphs that are to be read right to left.
On the far right is when the two atoms are not all up in each others business. Picture these atoms as magnets with the same poles trying to touch. They don't want to touch each other, but when you force them together they touch, which is the Same with these atoms. Something needs to put energy in for these guys to get together. As more energy is added, more potential energy builds up waiting to be released. so the more negative the y axis gets, the more potential energy there is going on in the bond. the far left is the potential energy being released and trickled off.
Guys, without a doubt this is helping me understand better than I expected, but this post is getting very long, so I am going to stop here and go to bed sometime soon. I bolded most of the important info so if you didn't want to read all of it i totally get it. If you did read it, I am clapping for you... because it was a lot. Alright, I hope your brains don't explode. Have a wonderful rest of your third quarter and good luck with your grades
Sunday, March 6, 2016
I Liked This Unit
Well since I didn't have much else to post about I decided to share with you how I liked this unit. First it wasn't as difficult as the past two units so my brain and my grade are very happy about that. Second, I felt that electron configurations were similar to a puzzle we had to figure out and that was enjoyable. Lastly, the whole unit was a lot of memorization which I am fair at so overall I liked. That is all.
Test Reflection
The test was on friday, and I felt good on it. I am waiting for the grade to be put in, because like always I need a good grade. Studying went well and I felt like I knew what i was doing. Ugh, the suspense is killer.
I hope everyone did well, and I hope you have a stress free weekend.
Saturday, February 27, 2016
Spectroscopic Analysis of Co and Cr Lab
This lab was not as interesting as the flame because there was no fire but it wasn't horrible. We used a Spec20 to analyze Co and Cr at different wavelengths. We have yet to do anything with the data and this post may change once we do somethings with it. Good luck to Everyone.
Friday, February 26, 2016
Quiz Reflection
Fortunately this quiz went well. I have figured out a good way to study so I know the information. I need to do well on this quiz so I don't dig myself into a whole with my grade. I wish all of my classmates good focus in future studying and tests.
Thursday, February 25, 2016
Aqueous Solutions Retake
Thank the Lord that we had a retake... My grade may or may not have been disgraceful. So I was able regain my family's honor by getting a good(hehe you guys don't know this but i'm in computer apps class typing this and the keyboard doesn't like to type the letters "D" and "T" so when I typed the word good it left out the d which left the word as goo... I'm sorry I'm a dork an though that was funny)grade on the retake. My grade is not in a deep whole now guys don't worry.
Wednesday, February 24, 2016
Helpful Links
Heres some helpful Links that I have found.
Good luck to all on life.
Crash Course
Crash Course Again
I promise its worth is to watch crash course sometimes
How about some Khan Academy now
and now
and one more just to be safe
Good luck to all on life.
Crash Course
Crash Course Again
I promise its worth is to watch crash course sometimes
How about some Khan Academy now
and now
and one more just to be safe
Friday, February 19, 2016
Flame Test Lab
This was by far my favorite lab of all this year. We got to light things on fire and watch them turn colors. It was great. We then took the color of the flame and used it to find the wavelength of the light that was burning on the small stick we were holding. After that, we used the wavelength and the cool equations we all learned to find the energy. Overall, interesting and awesome lab.


Saturday, February 13, 2016
The Hardest UInit
Well it was hard.... and the test sucked.... I didn't do well... life goes on... **crying**
Preparing for the Acid Base Test
So my friends.... ahead of us is the hardest test of the year. How might one prepare for this you might ask? Well, my inquisitive friend the answer lies in the hours of studying and reading an writing that have been put in already. You do packets over multiple times until you could tell someone both the questions and the answer. You read the book an do every question. You use every source Frank has to help.(It still didn't get me a good grade... I'm not salty at all) Well before I retreat into the depths that is chemistry again, I will wish you all luck on the rest of our semester and all future chem we may have.
Monday, February 8, 2016
Helpful Links
Crash Course
Khan Academy(This one and the ones after it go with Mrs. Frankenberg's Titration Calculation worksheet that she did not have a key for)
Molar Mass of an Unknown Acid
This lab is very similar to the Percent acid in vinegar lab, except we were to write our own procedure and given a little bit of a different task. As you can probably guess from the title, we are to find the molar mass of an unknown acid. This means more fancy titrations and for my group, re-standardizing and practically starting all over. Its totally cool though because I enjoy titrations.
We had this fancy way of stirring our water with our unknown acid. It was a small stir rod dropped in the flask, while the flask was sitting on a hot plate. A magnet in the hotplate caused the stir rod to spin which helped us dissolve our solution, along with the heat from the hot plate.
(There was a video, but I don't know how to do cool internet stuff so you can see it... I'm sorry. I hope that the description is enough... or the fact that you also participated in the lab)
Friday, February 5, 2016
Percent Acid In Vinegar
This lab was pretty rockin'. This was our first lab with our new partner, and I was happy with who I was paired with. We work well together and get things done. This lab was about titrating acids and bases in order to find the percent acid in vinegar. We finished this lab with a .85% error, which I cannot complain about. Here are some cool pictures from the lab. We have a titrated solution in between my partner and I in our natural habitat, and a buret filled with base.




Friday, January 29, 2016
Vitamin C Lab
Our first lab leading into the famously difficult acid-base unit was the Vitamin C lab, which I liked a lot. It was all about mixing a fruit juice together with starch. Then you counted the amount of drops of iodine it took to permanently change the color or the initial solvent. The funny thing was when you dropped the iodine in, it seemed to disappear and when it did change color it was wuite rapidly. My partner and I did five trials to recieve the best average we could.




Cool pictures yay!
Friday, January 15, 2016
More Aqueous Solutions Notes
The next step in aqueous solutions is learning how to integrate what we learned into stoichiometry. It's pretty much the same systematic type of math as the stoic unit but with another added conversion on there. I personally like stoichiometry, but I'm a dork so that could be why.
Here is an example of a question that i took in m notes. The math is no that bad but there are many rooms for mistakes.
Good luck, and successful studying.
Helpful Links:
Murder Molarity Lab
This was a pretty rad lab. Our good friend Mrs. Scarlet was murdered and as a forensic scientist it was our job to figure out who was the killer. First, we had to figure out what the murder weapon was by undergoing a reaction between the unknown substance that killed Mrs. Scarlett and Na2CO3. The end result of this reaction clears up the mystery of the murder weapon. If the reaction formed a precipitate then we knew that the murder weapon was AgNO3, but if it did not form a precipitate then the murder weapon is KI. The reaction formed .094g of precipitate so we know he murder weapon was AgNO3. To find out who the murderer was we needed to find the molarity of the substance which turned out to be .227 which told us the murderer was professor plum.
Thursday, January 7, 2016
First Aqueous Solutions notes
We fit a lot of information into one set of notes, but yet I don't feel that overwhelmed. We learned % mass, molarity and concentration of ions
Here is a link to a crash course solution that I thought helped.
Click Hither
Some random things to remember that i got down that might now be to the main notes:(please correct me if I don't have certain details correct)
Here is a link to a crash course solution that I thought helped.
Click Hither
Some random things to remember that i got down that might now be to the main notes:(please correct me if I don't have certain details correct)
- If a substance on a solubility curve has a negative slope, it is most likely a gas.
- Hydrogen bonds with only certain substances when in a long chain. the pneumonic for those substances is "phone call" or spelled F.O.N. Cl*. (the chlorine is only if it is terminal meaning on the end of the chain)
- Like dissolves like, polar dissolves polar, non-polar dissolves non-polar, ionic dissolves similarly to polar substances.
- When stating the answer in concentration of ions problems, state the charge in your answer if it is needed.
- (Volume of L)(Mol/L)=moles
- i(supposed to be cursive) is the Van't Hoff factor, which is the number of ions a substance will break apart into.
- Van't Hoff affects the melting/boiling/freezing point of solutions.
- Van't Hoff of covalent compounds is 0 because they do not ionize.
- you cannot have a Van't Hoff of zero, it starts at one.
That turned into a lot, but there it is.
Have a wonderful night and good luck with the studying
.
 |
| https://s-media-cache-ak0.pinimg.com/236x/d2/83/17/d28317cd998cb75b306d8bcb02f767a4.jpg |
Subscribe to:
Comments (Atom)